"None of my inventions came by accident. I see a worthwhile need to be met and I make trial after trial until it comes. What it boils down to is one per cent inspiration and ninety-nine percent perspiration." - Thomas Edison
"If Edison had a needle to find in a haystack, he would proceed at once with the diligence of the bee to examine straw after straw until he found the object of his search... I was a sorry witness of such doings, knowing that a little theory and calculation would have saved him ninety per cent of his labor." - Nikola Tesla
(Don't these quotes perfectly illustrate the difference between screening and de novo design?)
Over 100 years ago Emil Fischer, later a winner of the Nobel Prize in Chemistry, proposed an explanation for the observed specificity of an enzyme toward its respective substrate: according to his rationale, the substrate should fit the enzyme like a key fits its lock. With the advent of computers this simple observation provided the foundation for the most rational approach among drug discovery methods: the structure-based design. Our proprietary molecular design technology relies on the Monte Carlo scheme, which automatically generates compatible, synthetically available peptides within the "evolutionary constraints" provided by the structure of the receptor, the composition of the library of amino acids, the self-compatibility of the generated molecular assembly, and certain other, user-imposed preferences.
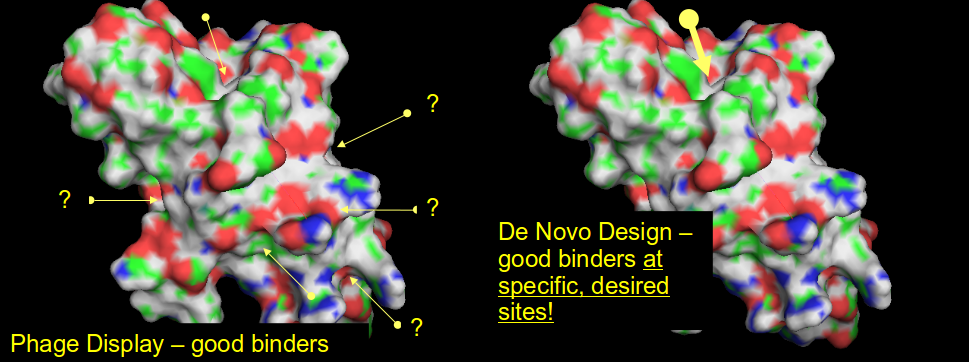
There are peptides and peptides, of course! Our design protocol is free from the inherent limitation of the currently predominant phage display approach - de novo design is capable of freely combining the natural amino acids with non-proteinogenic ones, and incorporating post-translational modifications. We are particularly interested in conformationally and proteolytically stable macrocyclic (head-to-tail) peptides. Such peptides deliver the functionality of biologics in a much smaller package.
Even more importantly, phage display delivers good binders, but nothing can be said about where these peptides bind and exactly how, whether they are agonists, antagonists or even if they possess any biological activity at all; these questions mandate further mechanistic and structural studies. Conversely, peptides designed de novo to interact with specific binding sites do precisely this: bind where they are expected to bind, and exert the desired biological effects.
Page by PGC
